Bienvenido al futuro de la TeleSalud
Combinamos innovación, tecnología y asistencia especializada para brindar la más óptima experiencia de usuario
Apostamos por una salud inclusiva y eficiente
En TeleMedik promovemos una salud equitativa, igualitaria y de fácil acceso. Ofrecemos soluciones de vanguardia que incorporan productos y procedimientos innovadores, adaptados a las distintas las poblaciones que servimos. Brindamos servicio de asistencia 24/7 todos los días del año.
+26 años de experiencia
ofreciendo soluciones de salud
+40 líneas de servicio
con asistencia bilingüe
+500 profesionales
de múltiples disciplinas
+14 softwares registrados
diseñados para brindar un servicio completo
Estamos contratando
Crece con nosotros
Conoce los beneficios de trabajar en TeleMedik y únete a nuestro equipo de profesionales multidisciplinarios especializados en los campos de salud y seguridad personal.
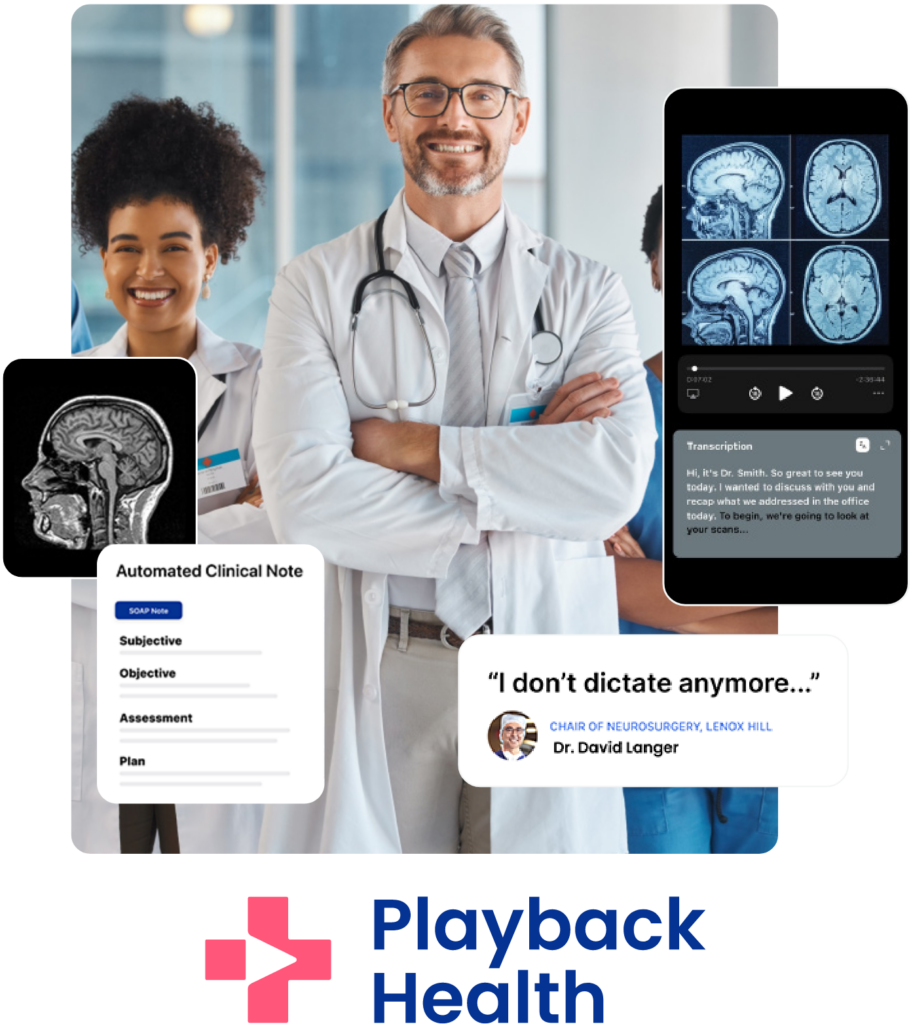
¡Le damos más tiempo al cuidado!
A través de la plataforma de Playback logramos un ahorro de 15 minutos en la documentación en EMR por paciente y un 20% de aumento en la productividad.
Tecnología unida al cuidado del paciente
Tenemos el potencial de conectar nuestras soluciones para ofrecer una experiencia única y completa

Conectamos al paciente a nuestra línea de enfermería (NAL) para evaluación clínica. ¡Con asistencia médica directa a través de vídeo consulta!

Sistema de aviso de emergencias con servicio de asistencia 24/7. Disponible en SDK para la integración en una aplicación móvil ya existente.

Un programa de monitoreo remoto de pacientes que combina tecnología con una metodología enfocada en las mejores prácticas de coaching.

Te ayudamos a implementar equipos de evaluación médica virtual en tu negocio. ¡Conoce la eficiencia en telemedicina!

Sistema de monitoreo remoto de pacientes basado en modelo predictivo (ACG). Se ajusta a las distintas poblaciones según las condiciones de salud y su complejidad.

Una nueva herramienta de transcripción basada en inteligencia artificial que convierte el audio y las conversaciones en notas clínicas en segundos.
![]()
Conectamos al paciente a nuestra línea de enfermería (NAL) para evaluación clínica. ¡Con asistencia médica directa a través de vídeo consulta!
![]()
Sistema de aviso de emergencias con servicio de asistencia 24/7. Disponible en SDK para la integración en una aplicación móvil ya existente.
![]()
Un programa de monitoreo remoto de pacientes que combina tecnología con una metodología enfocada en las mejores prácticas de coaching.

Te ayudamos a implementar equipos de evaluación médica virtual en tu negocio. ¡Conoce la eficiencia en telemedicina!
![]()
Sistema de monitoreo remoto de pacientes basado en modelo predictivo (ACG). Se ajusta a las distintas poblaciones según las condiciones de salud y su complejidad.
![]()
Una nueva herramienta de transcripción basada en inteligencia artificial que convierte el audio y las conversaciones en notas clínicas en segundos.
Enfocamos nuestros servicios hacia un modelo de salud híbrido
Interrelacionamos nuestras soluciones con programas de salud virtual que añaden valor en la prevención, diagnóstico, tratamiento y seguimiento de pacientes de forma personalizada.
Disponemos de distintos programas especializados en medicina, nutrición y dietética, manejo de condiciones crónicas, asistencia en emergencias y más.
Clientes y socios de negocio
Empresas que confían en nosotros
Mayor precisión mejores resultados
Experiencia Omnicanal
El Centro de Contacto de TeleMedik está dotado de tecnología de vanguardia para ofrecer un servicio de óptima calidad y satisfacción. Operamos bajo una estrategia de comunicación integrada que nos permite focalizarnos en procesos exclusivos para cada cliente.
Vídeo consulta de atención remota
para evaluación de enfermería (NAL) y visita médica en línea.
Consultas virtuales de médico a médico
para un mejor diagnóstico, tratamiento y derivación a especialistas.
Comunicación con los pacientes a través de su historial de salud
que incluye evaluaciones, resultados y contacto directo vía telefónica, correo electrónico y SMS.
Monitoreo a distancia de pacientes
bajo programa personalizado y llamadas de seguimiento.
Prescripción de medicamentos o prácticas médicas
con orientación y apoyo al paciente.
Recolección y revisión de datos biométricos mediante
periferales de salud y dispositivos portátiles (wearables) para la toma de signos vitales..
Certificaciones
Estándares y requisitos regulatorios que guían nuestros servicios
Experiencia de nuestros colaboradores







Nuestros expertos hablan
Conoce más sobre nuestras soluciones en el canal de YouTube de TeleMedik
¿En qué podemos ayudarte?
Estamos listos para ayudarte a encontrar soluciones innovadoras en pro del bienestar de escuelas, universidades, asociaciones, beneficiarios, organizaciones, proveedores de salud, entre otros grupos.


